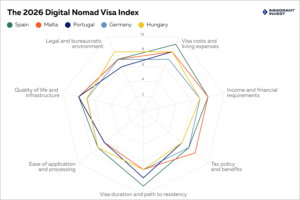
Mandara Biopharma Inc. announced approval from the FDA to initiate their clinical trials for the treatment of agitation associated with Alzheimer’s disease.
BOULDER, CO, UNITED STATES, February 17, 2026 /EINPresswire.com/ — Mandara Biopharma Inc., a privately held U.S.-based biopharmaceutical company focused on novel therapies for central nervous system (CNS) disorders, today announced that it has received approval from the U.S. Food and Drug Administration (FDA) to initiate their clinical trials for the treatment of agitation associated with Alzheimer’s disease.
Agitation is one of the most challenging and burdensome symptoms associated with Alzheimer’s disease, placing significant emotional, physical, and financial strain on caregivers, long-term care facilities, and healthcare systems. Agitation is common in Alzheimer’s disease, with approximately 60% of individuals with mild cognitive impairment and 76% of patients with Alzheimer’s dementia experiencing symptoms. In institutional settings, agitation is a major driver of increased costs, staff workload, and diminished quality of life, accounting for an estimated 2.9% to 6.1% of total institutionalization costs related to Alzheimer’s disease, which were estimated at $84.1 billion in 2018.
“Launching the clinical trials is an important milestone for Mandara as we advance a potential new therapeutic option for one of the most difficult aspects of caring for patients with Alzheimer’s disease,” said David Hurley, CEO of Mandara Biopharma. “Our goal is to bring forward a rigorously studied, FDA-approved treatment that can meaningfully improve outcomes for patients, caregivers, and care facilities.”
About Mandara Biopharma Inc.
Mandara Biopharma Inc. is a privately held U.S.-based biopharmaceutical company focused on novel therapies to treat CNS diseases. Their product pipeline includes new therapies for Alzheimer’s Agitation (2027 scheduled launch), Traumatic Brain Injury (2029) and PTSD (2030). Learn more at www.mandarabiopharma.com.
Forward-Looking Statements
This press release may include forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. The words “anticipate,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “project,” “will,” and similar expressions may identify forward-looking statements. Actual results may differ materially due to risks and uncertainties beyond the companies’ control. These statements are based on information currently available, and the companies assume no obligation to update such statements except as required by applicable law.
1 Lim MM, Gerstner JR, Holtzman DM. The sleep-wake cycle and Alzheimer’s disease: What do we
know? Neurodegenerative Disease Management 2014; 4(5): 351-362
2 Cloutier, M, et al. Institutionalization risk and costs associated with agitation in Alzheimer’s disease, Alzheimer’s and
Dementia: Translational Research and Clinical Interventions 5 (2019, 851-861)
David Hurley
Mandara Biopharma Inc.
+1 808-497-7771
dhurley@mandara.co
Visit us on social media:
LinkedIn
Instagram
Legal Disclaimer:
EIN Presswire provides this news content “as is” without warranty of any kind. We do not accept any responsibility or liability
for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this
article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
![]()